Đó là tiêu đề của báo cáo được đăng tải trên tạp chí Y khoa The New England Journal of Medicine (NEJM) ngày 09 tháng 12 năm 2017 và được trình bày tại hội nghị Huyết học Hoa Kỳ ngày 10 tháng 12 năm 2017 tại Atlanta.

Báo cáo này dựa trên một nghiên cứu về liệu pháp gen cho dạng bệnh hemophilia phổ biến nhất, do hãng dược phẩm BioMarin phát triển. Một tuần trước đó, một liệu pháp điều trị gen khác, được phát triển bởi sự hợp tác của hãng Spark Therapeutics và hãng Pfizer, cho một dạng ít phổ biến hơn của bệnh hemophilia cũng đã được công bố trên NEJM minh chứng cho phát biểu của Tiến sĩ y khoa Christiana Bardon, giám đốc của công ty đầu tư mạo hiểm MPM Capital và quỹ đầu tư mạo hiểm Burrage Capital: “Dữ liệu về liệu pháp gen đang trở thành “hiện tượng”.”
Bệnh hemophilia là bệnh dễ chảy máu (máu khó đông) do thiếu (hay bất thường) các yếu tố đông máu, bệnh có tính di truyền và từ lâu đã luôn là một nỗi ám ảnh của ngành y bởi những vết thương tưởng chừng nhỏ nhất cũng có nguy cơ cướp đi sinh mạng của bệnh nhân. Hiện nay, khoa học biết tới hai dạng hemophilia, cả hai đều xảy ra gần như hoàn toàn ở nam giới. Hemophilia A phổ biến hơn, là kết quả của sự thiếu hụt yếu tố đông máu VII xuất hiện ở khoảng 16.000 nam giới Hoa Kỳ và hơn 320.000 nam giới toàn cầu, theo số liệu của Hiệp hội Hemophilia Hoa Kỳ. Nghiên cứu của hãng BioMarin trên 9 bệnh nhân này cho thấy tỷ lệ chảy máu hàng năm của họ giảm từ khoảng 16 đợt xuống chỉ còn 1 đợt.
Ý tưởng thực hiện nghiên cứu đến từ nhà nghiên cứu dược phẩm Glenn Pierce, một bệnh nhân Hemophila, khi ông tình cờ phát hiện ra căn bệnh của mình được chữa khỏi nhờ ghép gan. Hemophilia B, là kết quả của sự thiếu hụt yếu tố đông máu IX, xuất hiện ở 4.000 người Hoa Kỳ và khoảng 80.000 nam giới toàn cầu. Các kết quả của nghiên cứu phối hợp bởi Spark Therapeutics và Pfizer trên dạng bệnh này giúp giảm tỷ lệ xuất huyết trung bình từ 11,1 lần xuống chỉ còn 0,4 lần mỗi năm.
Từ Forbes
(theo Namud Insider)
= = = = = = = = = = = = = = = = = =
Bài báo gốc Tiếng Anh:
Excitement Builds Around Gene Therapy Cures For Hemophilia
The New England Journal of Medicine declares a cure for hemophilia is near.THE NEW ENGLAND JOURNAL OF MEDICINE
How enthralled are scientists by the prospects for gene therapy in hemophilia, the deadly disease of uncontrolled bleeding? Ask the usually staid New England Journal of Medicine, which just ran this headline: “A Cure for Hemophilia [is] within Reach.”
It would be one of medicine’s holy grails – and they are the talk of the annual meeting of the American Society of Hematology here in Atlanta.
The New England Journal headline – which would have seemed fantasy just a few years ago – is a harbinger of a revolution in hemophilia treatment. A new drug, from Roche, could transform the way many patients are treated. But it’s being followed by gene therapy treatments that use viruses to implant new genes into patients’ cells, potentially making it so they no longer need any treatment. It’s a revolution that’s been a long time coming: The World Health Organization forecast that hemophilia gene therapy was imminent in 1994, and doctors made similar claims to Bloomberg News in 1999. Widespread internet use, the iPhone, and reality TV all happened in the interim. A cure for hemophilia didn’t.
Today’s excitement is driven by a study of a gene therapy for the most common form of hemophilia, developed by BioMarin of Notaro, Calif., published in the New England Journal and presented at ASH today, and another of a gene therapy treatment, from Spark Therapeutics and Pfizer, for a less common form of hemophilia, that the journal published last week. Other companies are following those leaders. Says Christiana Bardon, a manager at the venture capital firm MPM Capital and the hedge fund Burrage Capital: “The gene therapy data are looking phenomenal.”
How good were the gene therapy results? “The easiest thing to say is that we were absolutely blown away by them,” says K. John Pasi, of Barts Health NHS Trust and the London School of Medicine and Dentistry, who led the BioMarin study, of 9 patients. The patients all stopped using clotting factors to treat their hemophilia, yet their annualized bleeding rate fell from 16 episodes a year to just one. “Their bleeding rates collapsed to zero or nearly zero and we’ve improved their quality of life beyond recognition,” Pasi says. For the Spark gene therapy, bleeding rates were reduced from 11.1 bleeding events per year to 0.4 bleeding events per year.
“It’s a remarkable gift to be able to go from thinking about hemophilia every minute of every day to no longer worrying,” Pierce says. “If I stand up from this chair and I feel a little pain in my ankle, I know it is arthritis from all my bleeding episodes. It’s not bleeding. It’s a remarkable change in outlook, in independence, in freedom, to be able to now function without having to think of my hemophilia. That’s what a cure will mean for individuals who are able to achieve that through gene therapy.”
Hemophilia has long been a terrifying disease. Even small cuts and bruises can be deadly, and routine movement can lead patients to bleed into their joints, destroying them. This is the disease that so terrified the last Russian Tsarina that she sought the care of a strange mystic, Rasputin, to care for her son, Alexei. Her faith in a strange mystic helped undermine faith in the royal family, helping lead to the revolution that led to their deaths.
Scientists now know there are two forms of hemophilia, caused by defective genes for two different clotting factors needed to allow blood to coagulate. Both occur almost entirely in men. The more common form, hemophilia A, results from low levels of Factor VIII. It afflicts 16,000 men in the U.S., and more than 320,000 globally, according to the National Hemophilia Foundation. Hemophilia B, caused by a deficiency of clotting factor IX, afflicts about 4,000 Americans and 80,000 men globally. The BioMarin gene therapy treats hemophilia A; the Spark’s results on Thursday were for hemophila B.
Care of hemophiliacs has been transformed by the ability of companies to derive Factor VIII and Factor IX from blood products, and through genetic engineering. These have become multibillion-dollar products, sold by Shire, Novo-Nordisk, and Bioverativ.
One of the problems with these products is that some patients develop antibodies to their clotting factors. These can cause allergic reactions. It also means the clotting factors no longer work. These antibodies are called ‘inhibitors’, and 30% of hemophilia A patients develop them at some point. The usual treatment is to give clotting factor more often, to basically blow past the B cells that are producing the antibodies. This usually works, but in 5% to 10% of patients, other medicines must be used. The annual cost of factors for most hemophilia patients is $400,000 a year, according to Cowen & Co., but for inhibitor patients it is $1 million per patient. This summer, an Iowa insurer blamed its exit from the exchanges run under the Affordable Care Act on a teen with hemophilia whose treatment was costing $1 million a year.
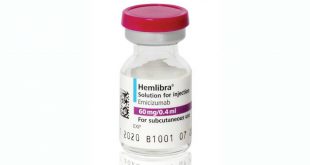
On Nov. 16, the FDA approved Hemlibra, an antibody drug, to treat hemophilia A patients who have antibodies against factor VIII. Its annual list price: $482,000. Factor VIII’s job is to bind together two other clotting factors: factor X and factor IXa. Hemlibra doesn’t resemble factor VIII at all, but mimics this function. The drug is approved for these patients already. Roche also said in November that Hemlibra was superior to factor VIII in patients with hemophilia A whether they had antibodies or not, but the company has yet to approve these results.
If there is a gene therapy revolution about to hit, these patients with inhibitors will have to wait in a long line. The BioMarin study, aside from only including adult patients, did not allow any patients with inhibitors. The reason is that one worry about gene therapy is that it will create antibodies. So far, it hasn’t. But there’s a question of whether gene therapy will work in these patients. It might. After all, says Pierce, the current treatment is to give more factor VIII. “Let’s just build a little bioreactor in the body and build constant amounts of Factor VIII,” he says, “and that would be an excellent way to tolerize a patient to gene therapy.”
But no company is likely to take that step until a product is approved for patients without inhibitors, Pierce says. BioMarin says it conduct two trials of its hemophilia A gene therapy, using slightly different doses. One will begin late this year, and a second early next, with both taking 52 weeks. After that, the company plans to ask for regulatory approval. Analysts think a competing hemophilia A gene therapy from Spark is just six months to a year behind.
 Câu lạc bộ bệnh máu khó đông bệnh viện quận Thủ Đức Câu lạc bộ bệnh máu khó đông bệnh viện quận Thủ Đức
Câu lạc bộ bệnh máu khó đông bệnh viện quận Thủ Đức Câu lạc bộ bệnh máu khó đông bệnh viện quận Thủ Đức